Introduction
Certain complications of pregnancy arise specifically during gestation, while others may preexist but impact the maternal health during pregnancy. These complications act as impediments to a healthy pregnancy, requiring healthcare professionals to navigate them skillfully for the well-being of both the mother and the unborn child. Pregnancy-induced hypertension (PIH), also known as gestational hypertension or pregnancy-related high blood pressure, is a condition that demands vigilant monitoring and expert nursing care.
This article aims to furnish nursing professionals with a comprehensive guide, offering insights into the understanding, identification, and management of pregnancy-induced hypertension. By exploring the pathophysiology, risk factors, and clinical manifestations of PIH, our goal is to empower nurses with the knowledge and skills necessary to deliver optimal care to mothers grappling with this challenge.
Pregnancy-induced hypertension
Pregnancy-induced hypertension (PIH), also known as gestational hypertension, is characterized by vasospasms occurring in both small and large arteries during pregnancy. This condition, occurring in approximately 5 to 8 percent of all pregnancies, manifests as high blood pressure and brings about an increase in the resistance of blood vessels. Consequently, there is a risk of compromised blood flow in various organ systems of the expectant mother, including the liver, kidneys, brain, uterus, and placenta.
Initially termed toxaemia, this condition was once thought to involve the production of a toxin by the woman in response to the foreign protein of the developing fetus. Researchers theorized that this toxin led to the typical symptoms associated with PIH. However, it’s noteworthy that no such toxin has been identified in connection with pregnancy-induced hypertension.
Pathophysiology of pregnancy-induced hypertension
The pathophysiology of pregnancy-induced hypertension (PIH) involves intricate changes in vascular dynamics during pregnancy. While the exact cause remains unclear, the development of PIH is attributed to factors such as vasospasms, which lead to sudden constrictions in both small and large arteries, resulting in increased resistance to blood flow. This heightened vascular resistance contributes to elevated blood pressure, placing additional strain on the cardiovascular system. Endothelial dysfunction, characterized by an imbalance in vasodilators and vasoconstrictors, further exacerbates vascular constriction.
The uterine arteries supplying blood to the placenta are particularly susceptible to these vasospasms, potentially causing placental insufficiency and compromising fetal oxygen and nutrient supply. PIH also impacts various organ systems in the mother, including the liver, kidneys, and brain, leading to potential complications such as impaired organ function. While precise triggers are not fully elucidated, identified risk factors include primigravida, multiple gestations, maternal age extremes, obesity, and a history of hypertension.
Understanding the pathophysiology of PIH is paramount for healthcare professionals to effectively monitor, manage, and mitigate associated risks during pregnancy, ensuring the well-being of both the mother and the developing fetus. Early detection and intervention play pivotal roles in optimizing outcomes for pregnant individuals with PIH.
Classification of pregnancy-induced hypertension
The classification of pregnancy-induced hypertension (PIH) involves categorizing the condition based on the severity of the associated blood pressure elevations and the presence of additional complications. The classifications commonly used are:
- Gestational Hypertension: This classification refers to elevated blood pressure that occurs after 20 weeks of gestation without the presence of significant proteinuria (excessive protein in the urine). It is often a precursor to more severe forms of PIH and requires close monitoring to assess the risk of progression.
- Preeclampsia: Preeclampsia is characterized by elevated blood pressure occurring after 20 weeks of pregnancy, accompanied by significant proteinuria. In addition to hypertension and proteinuria, preeclampsia can involve complications such as edema, organ dysfunction (particularly affecting the liver and kidneys), and a risk of adverse outcomes for both the mother and the fetus.
- Eclampsia: Eclampsia represents the most severe form of PIH and is characterized by the occurrence of seizures (convulsions) in a pregnant woman with preeclampsia. It poses serious risks to both maternal and fetal health and demands immediate medical attention and intervention.
- Chronic Hypertension with Superimposed Preeclampsia: Some individuals enter pregnancy with pre-existing chronic hypertension. If they develop preeclampsia on top of their existing hypertensive condition, it is termed chronic hypertension with superimposed preeclampsia. This classification acknowledges the challenge of managing individuals with pre-existing hypertension who may be at an increased risk of developing preeclampsia during pregnancy.
These classifications assist healthcare professionals in determining the appropriate level of monitoring, intervention, and management for pregnant individuals with varying degrees of hypertension. Regular assessments of blood pressure, urine protein levels, and organ function are essential components of classifying and managing PIH to ensure the best possible outcomes for both mother and baby.
Risk factors
Several risk factors contribute to the development of pregnancy-induced hypertension (PIH), highlighting the need for careful monitoring and intervention for individuals with heightened susceptibility. Common risk factors include:
- First-time Pregnancy (Primigravida): Women experiencing their first pregnancy are at an increased risk of developing PIH compared to those who have had previous pregnancies.
- Multiple Gestations: The presence of twins, triplets, or higher-order multiple pregnancies elevates the risk of PIH due to increased demands on the circulatory system.
- Maternal Age: Both younger women (under 20 years old) and older women (over 40 years old) face an elevated risk of developing PIH during pregnancy.
- Obesity: Excess body weight, specifically a body mass index (BMI) above the normal range, is associated with an increased risk of developing PIH.
- History of Hypertension: Individuals with a pre-existing history of chronic hypertension are at a higher risk of experiencing PIH during pregnancy.
- Family History: A family history of PIH or hypertensive disorders during pregnancy can contribute to an individual’s susceptibility.
- Pre-existing Medical Conditions: Certain medical conditions, such as diabetes, renal disease, and autoimmune disorders, may increase the risk of developing PIH.
- Vascular Disorders: Women with pre-existing vascular disorders or conditions affecting blood vessels may have an elevated risk of developing PIH.
- Race and Ethnicity: Some studies suggest variations in the prevalence of PIH among different racial and ethnic groups, with African American women facing a higher risk.
- Interval between Pregnancies: A short interval between pregnancies, defined as less than two years, may increase the risk of PIH.
Understanding these risk factors is crucial for healthcare professionals in identifying individuals who may be more susceptible to pregnancy-induced hypertension. Early identification and proactive management tailored to each patient’s risk profile are essential for mitigating the impact of PIH on maternal and fetal health. Regular prenatal care and monitoring play pivotal roles in addressing these risk factors and promoting positive pregnancy outcomes.
Signs and Symptoms
Pregnancy-induced hypertension (PIH) manifests through various signs and symptoms that necessitate vigilant monitoring and timely intervention. Common indicators include:
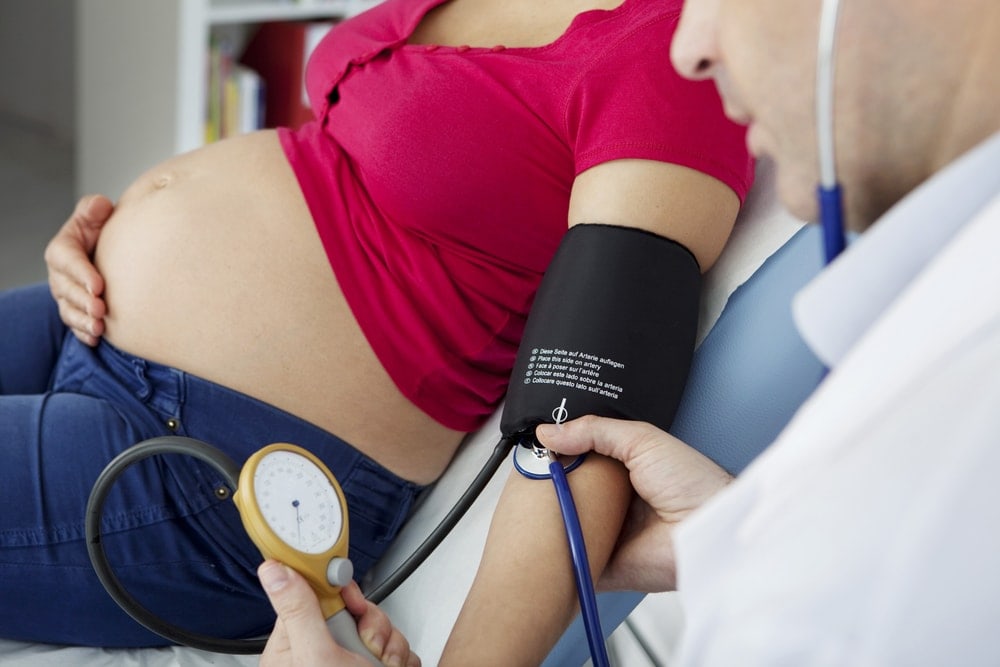
- Elevated Blood Pressure: One of the primary signs of PIH is an increase in blood pressure, typically occurring after 20 weeks of gestation. Hypertension is diagnosed when blood pressure consistently measures 140/90 mm Hg or higher.
- Proteinuria: Proteinuria, or the presence of excess protein in the urine, is a hallmark symptom of PIH. It is often identified through routine urine tests during prenatal care.
- Edema: Swelling, particularly in the hands, face, and legs, may occur in individuals with PIH. Edema is a result of fluid retention and increased vascular permeability.
- Headaches: Persistent headaches, often described as throbbing or pounding, can be a symptom of PIH. These headaches may not respond well to typical headache remedies.
- Visual Disturbances: Visual changes, such as blurred vision, light sensitivity (photophobia), or temporary loss of vision, can occur in severe cases and may indicate complications like preeclampsia.
- Abdominal Pain: Pain or discomfort in the upper abdomen, particularly on the right side, may signal liver involvement, a complication associated with severe PIH.
- Nausea and Vomiting: Some individuals with PIH may experience persistent nausea and vomiting, which may be indicative of more severe conditions like preeclampsia.
- Shortness of Breath: Difficulty breathing or shortness of breath can occur due to fluid retention and increased pressure on the lungs.
- Decreased Urination: Reduced urine output may be a sign of compromised kidney function, a potential consequence of severe PIH.
- Seizures (Eclampsia): In the most severe cases, PIH can progress to eclampsia, characterized by seizures. This is a medical emergency requiring immediate attention.
It’s crucial for pregnant individuals and healthcare professionals to be vigilant for these signs and symptoms, especially if risk factors for PIH are present. Regular prenatal check-ups, blood pressure monitoring, and urine tests are essential components of early detection and management. Prompt intervention is crucial to prevent complications and ensure the well-being of both the mother and the developing fetus.
Diagnostic tests
Diagnostic tests play a crucial role in identifying and monitoring pregnancy-induced hypertension (PIH), aiding healthcare professionals in timely intervention and management. Key diagnostic tests include:
- Blood Pressure Measurement: Regular monitoring of blood pressure is fundamental in diagnosing PIH. Elevated blood pressure, defined as consistently measuring 140/90 mm Hg or higher after 20 weeks of gestation, is a primary diagnostic criterion.
- Urine Tests for Proteinuria: Testing for proteinuria involves analyzing urine samples to detect the presence of excess protein. This helps confirm the diagnosis of PIH, with increased protein levels indicating potential complications.
- Blood Tests: Comprehensive blood tests may be conducted to assess organ function, including liver and kidney function. Abnormalities in these tests can indicate the severity of PIH and potential organ involvement.
- Complete Blood Count (CBC): CBC provides information about red blood cell count, white blood cell count, and platelet count. Abnormalities may suggest complications associated with PIH.
- Liver Function Tests: Evaluating liver enzymes, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST), helps assess liver function and detect potential complications like HELLP syndrome (Hemolysis, Elevated Liver enzymes, and Low Platelet count).
- Renal Function Tests: Blood tests, such as serum creatinine and blood urea nitrogen (BUN), help assess kidney function. Abnormal results may indicate impaired renal function, a possible consequence of severe PIH.
- Non-Stress Test (NST) and Biophysical Profile (BPP): Fetal monitoring through NST and BPP assesses the well-being of the fetus. These tests help determine fetal heart rate patterns and monitor amniotic fluid levels, providing insights into fetal health.
- Ultrasound Imaging: Ultrasound scans may be performed to assess the placenta, fetal growth, and amniotic fluid levels. Abnormalities may indicate complications associated with PIH.
- Doppler Ultrasound: Doppler ultrasound measures blood flow in the uterine and umbilical arteries, providing valuable information about placental function and potential restrictions in blood flow.
- 24-Hour Urine Collection: In cases of suspected severe PIH or preeclampsia, a 24-hour urine collection may be conducted to accurately measure protein excretion over a specified time period.
These diagnostic tests enable healthcare professionals to confirm the diagnosis of PIH, assess the severity of the condition, and identify any associated complications. Regular and thorough monitoring is crucial throughout pregnancy to ensure optimal maternal and fetal health.
Medical management
The medical management of pregnancy-induced hypertension (PIH) is focused on controlling blood pressure, preventing complications, and ensuring the well-being of both the mother and the fetus. The approach to medical management may vary based on the severity of PIH and individual patient factors. Key components of medical management include:
- Blood Pressure Monitoring: Regular monitoring of blood pressure is essential to track changes and assess the effectiveness of interventions. Individuals with PIH often require more frequent monitoring, and healthcare providers may recommend home blood pressure monitoring for additional oversight.
- Antihypertensive Medications: Antihypertensive medications may be prescribed to control elevated blood pressure. Common medications include methyldopa, labetalol, and nifedipine. The choice of medication depends on individual patient factors, such as gestational age, severity of hypertension, and potential side effects.
- Hospitalization: Severe cases of PIH may necessitate hospitalization for closer monitoring and management. This is particularly relevant if there are signs of complications, such as organ dysfunction or fetal distress.
- Magnesium Sulfate for Seizure Prevention: Magnesium sulfate may be administered to prevent seizures in individuals diagnosed with severe PIH or preeclampsia. This is particularly crucial in cases where there is an increased risk of progression to eclampsia.
- Corticosteroids: In cases of severe PIH, corticosteroids may be administered to enhance fetal lung maturity if early delivery is anticipated. This is often considered when the pregnancy needs to be terminated preterm to mitigate risks to both the mother and the fetus.
- Fetal Monitoring: Continuous fetal monitoring, such as non-stress tests (NST) or biophysical profiles (BPP), helps assess fetal well-being. Close monitoring is essential to detect any signs of fetal distress promptly.
- Delivery Timing: The decision regarding the timing of delivery is a critical aspect of medical management. In cases of severe PIH or complications, the healthcare team may recommend preterm delivery to safeguard the health of both the mother and the fetus.
- Postpartum Monitoring: Postpartum monitoring is crucial to assess blood pressure levels and ensure the resolution of PIH. Blood pressure management may continue after delivery, and close follow-up care is essential for ongoing evaluation.
- Fluid Balance: Maintaining optimal fluid balance is important in managing PIH. Healthcare providers carefully monitor fluid intake and output to prevent complications related to fluid retention or dehydration.
- Patient Education: Patient education is integral to medical management. Individuals with PIH are educated about the importance of regular prenatal visits, medication adherence, signs of complications, and when to seek medical attention.
Medical management of PIH requires a collaborative and individualized approach, with healthcare providers closely monitoring the patient’s condition throughout pregnancy and the postpartum period. Timely interventions and a multidisciplinary approach contribute to optimizing outcomes for both mother and baby.
Surgical management
Surgical management is not typically the first-line approach for pregnancy-induced hypertension (PIH). However, certain complications associated with severe PIH or preeclampsia may necessitate surgical interventions. The decision for surgical management is made on a case-by-case basis, considering the health status of both the mother and the fetus. Some scenarios where surgical interventions may be considered include:
- Cesarean Section (C-Section): In cases of severe PIH or preeclampsia, where there is a risk to the health of the mother or the fetus, a cesarean section may be recommended. This surgical delivery method allows for a controlled and expedited delivery, reducing the duration of exposure to the potential complications associated with PIH.
- Induction of Labor: If the mother’s blood pressure is difficult to control or if there are signs of fetal distress, healthcare providers may opt for the induction of labor to initiate childbirth. This is a controlled process that may be initiated with medications or other methods to bring about contractions and promote delivery.
- Placental Removal: In cases where the placenta is not functioning adequately due to compromised blood flow associated with PIH, surgical removal may be necessary. This can occur during a cesarean section or a postpartum procedure to address complications such as retained placenta.
- Tubal Ligation: In situations where a woman with severe PIH does not wish to have more children and has completed her family, a tubal ligation may be considered. This is a surgical procedure for permanent sterilization, often performed during a cesarean section or shortly after childbirth.
It is important to note that surgical interventions are reserved for specific circumstances where the benefits outweigh the risks. The decision for surgical management is typically made collaboratively between the patient, obstetrician, and other members of the healthcare team, taking into account the severity of PIH, the gestational age of the fetus, and the overall health status of the mother.
Continuous monitoring and careful consideration of the risks and benefits are crucial in determining the most appropriate course of action. The goal is to ensure the safety and well-being of both the mother and the fetus, addressing any complications associated with severe PIH in a timely and effective manner.
Nursing management
Nursing management plays a critical role in the care of individuals experiencing pregnancy-induced hypertension (PIH). The focus is on monitoring, supporting, and educating the pregnant individual to promote optimal outcomes. Here are key components of nursing management for PIH:
Assessment and Monitoring:
Regular monitoring of vital signs, including blood pressure, is fundamental. Nurses assess for signs and symptoms of PIH, such as proteinuria, edema, and other complications. Continuous fetal monitoring, such as non-stress tests (NST) and biophysical profiles (BPP), helps assess the well-being of the fetus.
Medication Administration:
Nurses administer antihypertensive medications as prescribed, closely monitoring the individual’s response and potential side effects. For severe cases, where magnesium sulfate is indicated for seizure prevention, nurses administer and monitor the infusion carefully.
Fluid and Electrolyte Balance:
Nurses monitor fluid intake and output to maintain optimal fluid balance. This is essential in preventing complications related to fluid retention or dehydration.
Patient Education:
Education is a key aspect of nursing management. Nurses provide information about the importance of prenatal care, medication adherence, signs of complications, and when to seek medical attention. Pregnant individuals are educated on self-monitoring of blood pressure at home if recommended by the healthcare provider.
Emotional Support:
Pregnancy-induced hypertension can be stressful. Nurses provide emotional support, addressing concerns and providing reassurance. Encouraging open communication helps pregnant individuals express their feelings and fears about the condition.
Positioning and Comfort Measures:
Nurses assist in positioning the pregnant individual to maximize comfort and minimize edema. Encouraging rest and providing comfort measures, such as applying cold compresses, can alleviate discomfort associated with edema.
Labor Support:
In cases where induction of labor or cesarean section is necessary, nurses provide support during the labor process. Continuous monitoring of both the mother and the fetus is maintained throughout labor and delivery.
Complication Prevention:
Nurses work proactively to prevent complications by closely monitoring for signs of deterioration in the mother’s condition. Close collaboration with the healthcare team ensures prompt intervention if complications arise.
Postpartum Care:
Nursing care extends into the postpartum period. Blood pressure monitoring continues, and nurses assess for any lingering signs of hypertension or complications. Patient education regarding postpartum recovery, signs of postpartum hypertension, and the importance of follow-up care is provided.
Interdisciplinary Collaboration:
Nurses collaborate with obstetricians, midwives, and other healthcare professionals to ensure comprehensive care. Communication between team members is crucial for providing individualized care based on the severity of PIH and the unique needs of each patient.
Nursing management for pregnancy-induced hypertension requires a holistic approach that addresses both the physical and emotional well-being of the pregnant individual. Regular assessments, vigilant monitoring, and effective communication contribute to optimal outcomes for both the mother and the newborn.
Read more