Chronic bronchitis challenges clinical teams daily across medical-surgical and pulmonary units. A structured, evidence-informed bronchitis nursing diagnosis care plan transforms complex respiratory compromise into measurable goals, restoring function, easing dyspnea, and preventing avoidable exacerbations. This guide presents a complete, practical framework assessment, NANDA nursing diagnoses, NIC interventions with rationales, NOC outcomes, and documentation optimized for learning and bedside application.
Nursing Care Plan for Chronic Bronchitis (NANDA, NIC, NOC)
Chronic bronchitis is a COPD phenotype defined by a productive cough for at least three months in two consecutive years, unrelated to other causes. The disease process involves:
- Persistent airway inflammation
- Goblet cell hyperplasia and mucus hypersecretion
- Ciliary dysfunction and impaired mucociliary clearance
- Airway wall thickening and remodeling
- Ventilation–perfusion (V/Q) mismatch leading to hypoxemia, and in advanced disease, hypercapnia
Common risk factors
- Tobacco smoke exposure (active or secondhand)
- Occupational dusts and chemical fumes
- Indoor biomass fuel exposure
- Recurrent respiratory infections
- Genetic predisposition (including alpha‑1 antitrypsin deficiency)
Clinical implications
- Frequent cough with sputum production
- Recurrent exacerbations, often triggered by infection
- Gradual activity limitation due to dyspnea and fatigue
- Potential progression to pulmonary hypertension and cor pulmonale
Comprehensive Assessment for a Chronic Bronchitis Care Plan
Subjective Data
- Chronic productive cough; sputum characteristics (color, volume, viscosity)
- Dyspnea pattern: mMRC grade, triggers, nocturnal symptoms
- Chest tightness, wheeze, fatigue, sleep disturbance
- Exacerbation history: frequency, severity, steroid/antibiotic use, hospitalizations
- Smoking status and quit history; exposure to irritants
- Medication adherence and inhaler technique confidence
- Nutrition and appetite; unintended weight loss
- Psychosocial factors: anxiety, depression, social support
- Vaccination status (influenza, pneumococcal, COVID‑19)
Objective Data
- Vital signs: RR, HR, BP, temperature, SpO2 at rest and with exertion
- Respiratory assessment: accessory muscle use, pursed‑lip breathing, tripod posture
- Breath sounds: wheezes, rhonchi, crackles; diminished bases
- Sputum quantity/quality; signs of purulence
- Cyanosis (lips, nail beds), digital clubbing (less common), peripheral edema (cor pulmonale)
- Weight/BMI, muscle wasting, cachexia
- Functional capacity: 6‑minute walk distance, desaturation on ambulation
- Inhaler devices present; return demonstration of technique (with teach-back)
Diagnostics and Monitoring
- Spirometry: FEV1/FVC < 0.70 post‑bronchodilator; severity staged by FEV1 %
- ABG: hypoxemia; hypercapnia in advanced disease
- Pulse oximetry: resting and exertional SpO2
- CBC: polycythemia in chronic hypoxemia; eosinophils to guide ICS
- Chest radiograph: increased bronchovascular markings; hyperinflation signs
- Sputum culture during infectious exacerbations
- BNP/echocardiogram if cor pulmonale suspected
- CAT (COPD Assessment Test) and mMRC dyspnea scale for symptom burden
Priority NANDA Nursing Diagnoses for Chronic Bronchitis
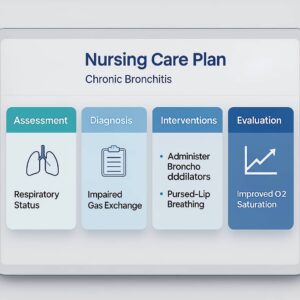
- Ineffective Airway Clearance related to excessive mucus and ciliary dysfunction
- Impaired Gas Exchange related to V/Q mismatch and alveolar hypoventilation
- Ineffective Breathing Pattern related to bronchospasm, anxiety, and fatigue
- Activity Intolerance related to imbalance between oxygen supply and demand
- Risk for Infection related to retained secretions and impaired defenses
- Imbalanced Nutrition: Less Than Body Requirements related to increased work of breathing and anorexia
- Deficient Knowledge (Disease Management, Inhaler Technique, Oxygen Safety)
- Anxiety related to dyspnea and chronic illness burden
- Disturbed Sleep Pattern related to nocturnal cough and hypoxemia
NOC Outcomes and SMART Goals
- Respiratory Status: Airway Patency
- Effective cough evident; sputum expectorated without distress
- Breath sounds clear or improved with reduced rhonchi
Gas Exchange
- SpO2 maintained at prescriber‑ordered targets (commonly 88–92% for CO2 retainers)
- ABG: PaO2 and PaCO2 stabilized toward baseline values
Breathing Pattern
- RR within target range; reduced accessory muscle use
- Reports of reduced dyspnea on mMRC scale
Activity Tolerance
- Ability to perform ADLs with minimal rest periods
- Improved 6‑minute walk distance with acceptable desaturation thresholds
Infection Severity
- Afebrile; sputum becomes less purulent; WBC stabilizes
Nutritional Status
- Weight stabilized or trending upward toward goal
- Adequate caloric/protein intake documented
- Knowledge: Disease Process and Regimen
- Accurate return demonstration of inhaler technique
- Verbalization of exacerbation action plan and oxygen safety
Anxiety Level
- Reduced anxiety scores; improved sleep
Nursing Interventions (NIC) and Rationales by Diagnosis
Ineffective Airway Clearance
Goals (NOC)
- Airway patency maintained; effective cough
- Sputum mobilized and cleared without excessive fatigue
Interventions and rationales
- Positioning with HOB elevated 30–45 degrees and alternating side‑lying and high‑Fowler’s enhances ventilation and secretion drainage via gravity and chest wall mechanics.
- Hydration via adequate oral intake as permitted and IV fluids when ordered—liquefies secretions and improves ciliary transport.
- Humidification with humidified oxygen or room air helps prevent mucus plugging by maintaining airway moisture.
- Breathing techniques such as huff coughing and diaphragmatic/pursed‑lip breathing move secretions proximally while reducing air trapping and prolonging exhalation.
- Airway clearance therapy CPT, percussion, vibration, and PEP devices coordinated with respiratory therapy mobilizes secretions from peripheral to central airways.
- Pharmacologic airway support with SABA/LABA and anticholinergics, plus mucolytics when ordered, enlarges airway diameter and reduces mucus viscosity.
- Nebulizer care that maintains device hygiene, ensures correct dosing, and monitors response optimizes medication deposition and therapeutic effect.
- Indicated oropharyngeal or nasopharyngeal suctioning removes retained secretions when cough is ineffective while minimizing hypoxemia risk.
- Energy pacing that schedules airway clearance 30–60 minutes before meals and before rest periods reduces fatigue and aspiration risk.
- Sputum monitoring for volume, color, and odor with cultures when infection is suspected enables early detection of infectious exacerbations.
Impaired Gas Exchange
Goals (NOC)
- SpO2 maintained at target; ABGs near baseline
- Reduced signs of hypoxia: improved mentation, less cyanosis
Interventions and rationales
- Oxygen therapy: Titrate oxygen to prescriber‑ordered targets (often 88–92% in COPD) to optimize oxygenation while minimizing CO2 retention.
- ABG/pulse oximetry monitoring: Check trends at rest and with activity to detect deterioration and guide therapy adjustments.
- Ventilatory drive assessment: Observe for somnolence, headache, and signs of CO2 narcosis to identify hypercapnia complications requiring prompt action.
- Bronchodilator scheduling: Ensure long‑acting agents are administered on schedule and rescue inhalers used for breakthrough dyspnea as ordered to maintain airway tone and reduce V/Q mismatch.
- Corticosteroid therapy: Administer systemic or inhaled steroids when ordered for exacerbations or frequent flares to reduce airway inflammation and edema.
- Positioning for gas exchange: Use High‑Fowler’s or tripod positioning as tolerated to improve diaphragmatic excursion and lung volumes.
- Early mobilization: Encourage early mobilization and ambulation with rest breaks to enhance ventilation and prevent deconditioning.
- Noninvasive ventilation: Consider NIV during acute decompensation, when ordered, to support ventilation, reduce work of breathing, and improve gas exchange.
Ineffective Breathing Pattern
Goals (NOC)
- RR within target range; diminished accessory muscle use
- Regular breathing pattern with reduced dyspnea
Interventions and rationales
- Breathing retraining: Coach diaphragmatic and pursed‑lip breathing during activity to improve ventilation efficiency and reduce dynamic hyperinflation.
- Anxiety–dyspnea cycle interruption: Use calm communication, guided imagery, or brief relaxation exercises to interrupt anxiety‑induced hyperventilation and air trapping.
- Bronchospasm control: Time bronchodilators before activity or airway clearance to maximize bronchodilation when ventilatory demand increases.
- Nocturnal hypoventilation/OSA evaluation: Assess for nocturnal hypoventilation or OSA and support sleep study referrals when indicated to treat sleep‑related breathing disorders and improve daytime function.
- Fan or cool airflow: Apply a handheld fan or cool airflow to the face, if tolerated, to stimulate trigeminal receptors and reduce dyspnea perception.
Activity Intolerance
Goals (NOC)
- Performs ADLs with minimal dyspnea
- Improved exercise tolerance on standardized tests
Interventions and rationales
- Activity pacing breaking tasks into steps and alternating rest with activity prevents fatigue and maintains oxygen supply–demand balance.
- Energy conservation strategies seated bathing, rolling carts, reachers, and arranging frequently used items within easy reach minimize unnecessary energy expenditure.
- Pre-medication with a bronchodilator 15–30 minutes before activity, if ordered, reduces exertional dyspnea.
- Referral to pulmonary rehabilitation provides supervised exercise and education that improve endurance and quality of life.
- Nutritional timing with small, frequent, nutrient-dense meals away from peak activity avoids post-prandial dyspnea and supports energy needs.
- Teaching and cueing pursed-lip breathing during exertion control RR and prevent air trapping.
Risk for Infection
Goals (NOC)
- Afebrile, clear sputum trends; no new infiltrates on imaging
- Reduced frequency and severity of exacerbations
Interventions and rationales
- Reinforce hand hygiene and cough etiquette to reduce pathogen transmission.
- Verify influenza, pneumococcal, and COVID‑19 immunization status and facilitate administration per protocol to prevent or attenuate respiratory infections.
- Optimize airway clearance (e.g., CPT, PEP, adequate hydration) to reduce mucus stasis that fosters bacterial growth.
- Practice antibiotic stewardship by obtaining sputum culture when indicated and administering antibiotics only when criteria are met to target therapy and avoid resistance.
- Implement oral care protocols to lower oropharyngeal colonization and aspiration risk.
- Enforce environmental controls by minimizing exposure to smoke, dust, and fumes to reduce airway irritation and exacerbation triggers.
Imbalanced Nutrition: Less Than Body Requirements
Goals (NOC)
- Weight stabilization or gain toward individualized target
- Meets protein and caloric requirements
Interventions and rationales
- A nutrition consult to tailor a high‑calorie, high‑protein plan to energy expenditure compensates for the increased work of breathing.
- Provide small, frequent meals with soft, easy‑to‑chew options to reduce dyspnea during meals and improve intake.
- Offer oral supplements between meals and monitor satiety and tolerance to enhance caloric density without large volumes
- Coordinate respiratory treatments outside meal times to prevent nausea and enhance appetite.
- Monitor weight, prealbumin, and intake trends to enable early detection of malnutrition and guide timely intervention.
Deficient Knowledge (Disease Management and Devices)
Goals (NOC)
- Accurate inhaler/nebulizer technique demonstrated
- Verbalization of action plan for exacerbations and oxygen safety
Interventions and rationales
- Inhaler technique training: Demonstrate and observe return demonstration for MDI with spacer, DPI, or nebulizer to maximize drug delivery.
- Regimen literacy: Provide a simple schedule distinguishing controller from rescue medications to prevent under‑ or overuse and reduce exacerbations.
- Exacerbation action plan: Teach early signs worsening dyspnea, sputum purulence, fever and a stepwise response per provider plan to enable timely action that shortens exacerbation duration.
- Oxygen safety: Educate on no smoking near oxygen, safe storage, and fire precautions to prevent catastrophic fire risk.
- Environmental control guidance: Provide allergen and irritant reduction strategies to lower exacerbation triggers.
Anxiety
Goals (NOC)
- Reports of reduced anxiety; improved sleep
- Demonstrates use of coping strategies during dyspnea spikes
Interventions and rationales
- Provide therapeutic presence and reassurance with clear, calm explanations to reduce sympathetic arousal that exacerbates dyspnea.
- Implement breathing‑focused relaxation (box breathing, paced respiration, mindfulness) to lower ventilatory drive and perceived breathlessness.
- Involve social support and counseling services when indicated to address chronic illness burden and isolation.
- Deliver sleep hygiene education regular schedule, minimized stimulants, and optimized nocturnal oxygen if ordered to promote restorative sleep that reduces anxiety and daytime fatigue.
Pharmacologic Overview and Nursing Considerations
Note: Medication choices align with prescriber orders and GOLD guidelines for COPD phenotypes; chronic bronchitis often benefits from dual or triple inhaled therapy and selected adjuncts.
- Short‑acting bronchodilators (SABA/SAMA): Albuterol, ipratropium
- Nursing: Monitor HR, tremor; space doses; teach appropriate rescue use.
- Long‑acting bronchodilators
- LABA: Salmeterol, formoterol
- LAMA: Tiotropium, umeclidinium
- Nursing: Emphasize daily adherence; watch for dry mouth, urinary retention with LAMA.
- ICS or LABA/LAMA/ICS combinations
- Indication: Frequent exacerbations, elevated eosinophils, asthma‑COPD overlap.
- Nursing: Rinse mouth after ICS; monitor for thrush and pneumonia risk.
- Phosphodiesterase‑4 inhibitor: Roflumilast for chronic bronchitis phenotype with frequent exacerbations and FEV1 < 50%
- Nursing: Monitor weight loss, GI effects, mood changes.
- Mucolytics/Expectorants: Guaifenesin; consider N‑acetylcysteine per protocol
- Nursing: Ensure hydration; evaluate effectiveness.
- Systemic corticosteroids (short course during exacerbation)
- Nursing: Monitor glucose, mood, infection risk; taper as ordered.
- Antibiotics for bacterial exacerbations (e.g., amoxicillin‑clavulanate, doxycycline, macrolides)
- Nursing: Review allergies, QT prolongation risk, and interactions.
- Vaccines: Influenza, pneumococcal (PCV/PPSV), COVID‑19
- Nursing: Verify eligibility and timing; document administration.
- Long‑term oxygen therapy (LTOT)
- Indications: Resting PaO2 ≤ 55 mmHg or SaO2 ≤ 88%; or PaO2 55–59 with cor pulmonale/polycythemia.
- Nursing: Educate on adherence (≥15 h/day when indicated), humidification needs, and safety.
Non‑Pharmacologic Interventions That Change Trajectory
Smoking cessation
Behavioral counseling combined with pharmacotherapy nicotine replacement therapy (patch, gum, lozenge), varenicline, or bupropion achieves the highest quit rates and slows FEV1 decline. A structured quit plan with trigger management, regular follow‑up, and medication support lowers exacerbation frequency and mortality risk.
Pulmonary rehabilitation
A multidisciplinary program integrating supervised exercise training, breathing retraining, and self‑management education improves dyspnea, functional capacity, and health status while reducing hospitalizations. Typical courses span 6–12 weeks with 2–3 sessions weekly, guided by individualized goals and outcomes such as 6‑minute walk distance and symptom scores.
Airway clearance techniques
Positive expiratory pressure (PEP) and oscillatory devices (e.g., Flutter, Acapella, Aerobika) mobilize mucus by creating back‑pressure and vibration within the airways. Autogenic drainage and scheduled clearance sessions are most effective when timed after bronchodilator therapy and adequate hydration; collaboration with respiratory therapy optimizes technique and tolerance.
Breathing techniques
Diaphragmatic breathing promotes abdominal excursion during inspiration, enhancing ventilation efficiency, while pursed‑lip breathing prolongs exhalation to reduce air trapping. Regular practice during exertion and recovery periods lowers dyspnea perception and can stabilize respiratory rate.
Nutrition optimization
High‑calorie, high‑protein nutrition counters the catabolic impact of increased work of breathing and supports lean body mass. Small, frequent meals, oral supplements, and dietitian‑directed plans help meet energy needs, especially when meals are scheduled away from peak fatigue or airway treatments.
Environmental modifications
Reducing exposure to tobacco smoke, indoor pollutants, dusts, and chemical fumes lessens airway inflammation and symptom flares. HEPA filtration, well‑fitting masks in polluted settings, and control of indoor humidity and temperature further minimize irritant burden.
Sleep optimization
Screening for obstructive sleep apnea and evaluation for nocturnal hypoxemia guide the need for CPAP, BiPAP, or nighttime oxygen per clinical orders. Consistent sleep routines, head‑of‑bed elevation, and avoidance of stimulants and heavy meals before bedtime improve sleep quality and next‑day function.
Psychosocial support
Breathlessness commonly coexists with anxiety and depression, amplifying symptom distress and limiting activity. Early access to counseling or cognitive‑behavioral therapy, relaxation techniques, peer support groups, and family education improves coping capacity, engagement with care, and overall quality of life.
Education and Self‑Management Essentials
Inhaler mastery
- MDI with spacer: Shake; exhale fully; seal lips; inhale slowly with actuation; hold breath 5–10 seconds; wait between puffs; rinse mouth after ICS.
- DPI: Load dose; exhale away; seal lips; inhale forcefully; hold breath; close device; rinse after ICS.
- Nebulizer: Correct dose; cleaning and air‑drying post‑use to prevent contamination.
Symptom monitoring
- Daily assessment of dyspnea, cough, sputum changes, and fatigue levels.
- Use of CAT or mMRC to quantify symptom burden.
Exacerbation action plan
- Recognize early warning signs (increased dyspnea, sputum purulence/volume, fever, reduced exercise tolerance).
- Implement prescriber‑approved step‑up therapy and seek evaluation when red flags occur (e.g., severe dyspnea at rest, cyanosis, confusion).
Oxygen safety and adherence
- No flames or smoking near oxygen; secure tanks; avoid petroleum‑based products near nasal cannula.
Vaccination adherence
- Maintain up‑to‑date immunizations to reduce infection‑related exacerbations.
Interdisciplinary Collaboration and Discharge Planning
Respiratory therapy
Respiratory therapy supports accurate spirometry by coaching maneuvers, verifying bronchodilator withholding when appropriate, and documenting effort quality. Services include inhaler/nebulizer device selection and technique training, delivery of airway clearance therapies (CPT, PEP, oscillatory devices), and noninvasive ventilation titration to relieve hypercapnia while monitoring comfort and gas exchange.
Pharmacy
Pharmacy conducts comprehensive medication reconciliation to remove duplications and interactions across respiratory and comorbid regimens. Support includes selecting suitable inhaler devices, counseling on adverse effects, and providing adherence aids such as spacers, dose trackers, calendars, and refill synchronization.
Dietetics
Dietetics establishes individualized caloric and protein targets to offset increased work of breathing and prevent muscle loss. Interventions emphasize small, frequent, nutrient‑dense meals, oral supplements when indicated, and timing nutrition away from airway treatments to reduce post‑prandial dyspnea.
Physical/occupational therapy
Physical and occupational therapy teaches pacing and energy conservation, refines task sequencing, and recommends adaptive equipment such as shower chairs, long‑handled reachers, and rolling carts. Structured exercise plans blend aerobic and resistance training with breathing retraining to improve endurance, balance, and safety during ADLs.
Social work and case management
Social work and case management secure access to home oxygen, medications, and durable medical equipment, and arrange transportation for clinic and rehabilitation visits. Coordination includes pulmonary rehabilitation enrollment, linkage to smoking cessation and mental health resources, and navigation of insurance or community assistance programs.
Discharge checklist
- Confirm correct inhalers and spacer provided; teach‑back completed
- Written action plan for exacerbations
- Oxygen prescription and safety review if applicable
- Follow‑up appointments scheduled (pulmonology/primary care/rehab)
- Vaccination plan and smoking cessation resources
Special Considerations
Older adults
- Increased risk of steroid side effects, osteoporosis, and polypharmacy interactions; fall‑prevention strategies during dyspnea episodes.
Comorbidities
- Heart failure: monitor fluid status; careful diuretic balance with respiratory status.
- Diabetes: steroid‑induced hyperglycemia surveillance.
- Anxiety/depression: regular screening and treatment coordination.
Alpha‑1 antitrypsin deficiency
- Consider testing in early‑onset COPD or minimal smoking history; augmentation therapy per specialist.
Exacerbations vs stable disease
- Intensify bronchodilators, add short steroid courses, and consider antibiotics during exacerbations; return to optimized maintenance after recovery.
Home vs hospital management
- NIV and controlled oxygen for hypercapnic acute exacerbations; early mobilization and rapid de‑escalation when stable.
Documentation Tips (Examples)
Assessment
- “RR 24, accessory muscle use present, SpO2 89% RA; diffuse expiratory wheezes, coarse rhonchi in bases; productive cough with thick yellow sputum, 25 mL this shift.”
Interventions
- “Pursed‑lip breathing coached during ambulation; SABA given via MDI with spacer; chest physiotherapy performed 15 minutes; encouraged fluid intake as tolerated.”
Response/Evaluation
- “Post‑treatment SpO2 91% on 2 L/min; decreased wheezes; effective huff cough demonstrated; patient reports dyspnea improved from 7/10 to 4/10.”
Education
- “Return demonstration of DPI technique accurate; verbalized understanding of action plan and oxygen safety precautions.”
Sample Care Plan Snapshot (Condensed)
| Nursing Diagnosis | Goals/Outcomes (NOC) | Interventions (NIC) | Rationale |
| Ineffective Airway Clearance | Effective cough; clear breath sounds | Hydration, humidification, huff coughing, bronchodilators, airway clearance therapy | Thin secretions, improve mucociliary function, mobilize mucus. |
| Impaired Gas Exchange | SpO2 at target; improved ABGs | Titrated O2, positioning, bronchodilators/ICS, monitor ABG | Reduce V/Q mismatch; prevent CO2 retention |
| Activity Intolerance | ADLs with minimal dyspnea | Pacing, energy conservation, pre‑medication, pulmonary rehab | Balance oxygen demand/supply; build endurance |
| Risk for Infection | Afebrile; sputum improves | Hand hygiene, vaccines, oral care, culture‑guided antibiotics | Reduce infection risk and exacerbation frequency |
| Imbalanced Nutrition | Weight stabilization/gain | Small frequent meals, supplements, dietitian referral | Offset increased metabolic demand |
Evaluation and Quality Metrics
- Symptom scales: Improvement in CAT score by ≥2 points; mMRC reduction
- Exacerbation rate: Fewer steroid/antibiotic bursts over 6–12 months
- Functional capacity: Longer 6‑minute walk distance; fewer rest breaks during ADLs
- Readmissions: Reduced 30‑day COPD readmissions with action plans and early follow‑up
- Safety: No oxygen‑related incidents; correct inhaler technique documented each visit
Common Pitfalls and Prevention
Inhaler misuse
Incorrect inhaler technique remains a leading cause of poor symptom control and unnecessary escalation of therapy. Prevention centers on routine technique checks with teach-back at every visit, plus device selection matched to hand strength, coordination, vision, and cognition. Spacers, pictorial step cards, and dose counters further support consistent, effective use.
Over-oxygenation in CO2 retainers
Excessive oxygen can worsen hypercapnia in susceptible COPD phenotypes through V/Q mismatch and the Haldane effect. Prevention includes clear, written SpO2 targets (commonly 88–92% when ordered), controlled delivery devices such as Venturi masks, and prompt reassessment after any flow change. Frequent spot checks, ABGs when indicated, and monitoring for somnolence or confusion help detect CO2 retention early.
Skipped vaccinations
Missed immunizations increase infection-triggered exacerbations and hospitalizations. Prevention relies on embedding vaccine screening in every encounter, using standing orders and registry checks to identify gaps. Offering influenza, pneumococcal, COVID‑19, and Tdap vaccines on-site with clear documentation improves uptake and continuity.
Deconditioning
Reduced activity accelerates muscle loss, worsens dyspnea, and limits independence. Prevention pairs early mobilization with structured progression: out-of-bed time, hallway ambulation, and staged resistance training guided by PT/OT. Activity pacing, pre-activity bronchodilator timing when ordered, and close SpO2 monitoring maintain safety while building endurance.
Polypharmacy errors
Complex regimens increase the risk of duplications, interactions, and missed doses. Prevention begins with thorough medication reconciliation and simplification to the fewest effective inhalers, ideally once-daily combinations when appropriate. Standardized schedules, color-coded labeling, and synchronized refills support adherence, while pharmacist review mitigates interaction risks and overlapping mechanisms.
Conclusion
A robust bronchitis nursing diagnosis care plan for chronic bronchitis balances pathophysiology, precision assessment, and practical interventions. When NANDA diagnoses are paired with clear NOC goals and NIC interventions backed by education, pulmonary rehabilitation, and vigilant monitoring patients experience fewer exacerbations, stronger functional capacity, and better quality of life. Consistent documentation, interdisciplinary teamwork, and adherence to evidence‑based protocols elevate outcomes across care settings.
FAQs: Chronic Bronchitis Nursing Care Plan
Q1: What are the top NANDA nursing diagnoses for chronic bronchitis?
Ineffective Airway Clearance, Impaired Gas Exchange, Ineffective Breathing Pattern, Activity Intolerance, Risk for Infection, Imbalanced Nutrition: Less Than Body Requirements, Deficient Knowledge, and Anxiety commonly guide the care plan.
Q2: What oxygen saturation targets are recommended for chronic bronchitis in COPD?
Many protocols target SpO2 88–92% for patients at risk of hypercapnic respiratory failure. Exact targets follow prescriber orders and institutional policy, with ABGs guiding adjustments.
Q3: Which nursing interventions most effectively improve airway clearance?
Hydration, humidification, huff coughing, diaphragmatic/pursed‑lip breathing, bronchodilators, mucolytics, and coordinated chest physiotherapy/PEP devices are core strategies. Suctioning is reserved for ineffective cough.
Q4: When is long‑term oxygen therapy indicated?
Typical indications include resting PaO2 ≤ 55 mmHg or SaO2 ≤ 88%, or PaO2 55–59 mmHg with cor pulmonale or polycythemia. Final decisions are made by prescribers based on comprehensive assessment.
Q5: How does pulmonary rehabilitation support chronic bronchitis care plans?
Pulmonary rehabilitation combines supervised exercise, breathing retraining, education, and psychosocial support, improving dyspnea, exercise tolerance, and health‑related quality of life while reducing exacerbations and hospitalizations.